Ryan Yu | 7/5/2025
The origins of electrochemistry were as spontaneous as the reactions it explores, over two hundred years ago in the University of Bologna, Italy. Originally a comparative anatomist, Luigi Galvani was examining the structure of a dissected frog when his metal instrument brushed the exposed nerves of the leg. The leg twitched.
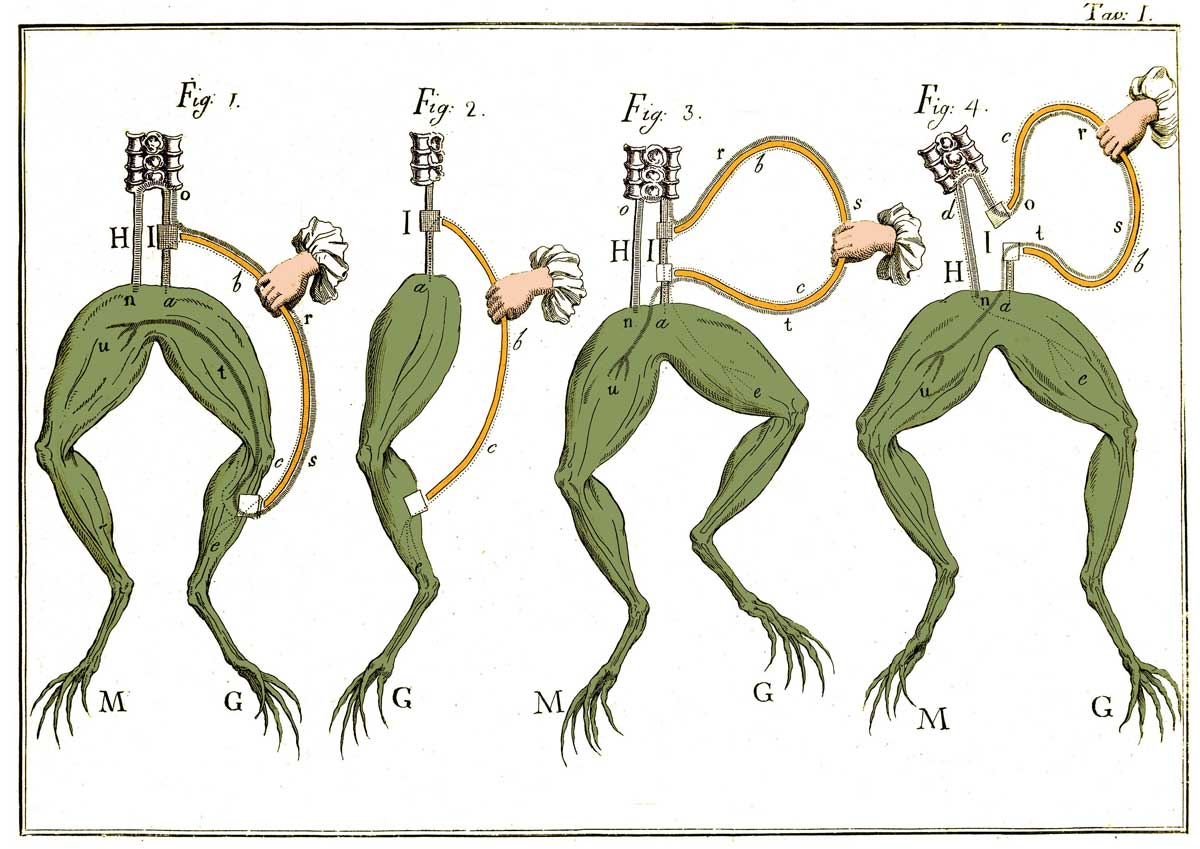
Intrigued, Galvani further investigated, and found that the legs would twitch when touched by different metals, which led him to describe the frog as holding “animal electricity.” He became obsessed with the idea that there was an electricity of life intrinsic to biological tissue. Had he not held an academic rivalry with colleague Alessandro Volta, scientists may have operated with this understanding for the next hundred years. Volta, however, demonstrated the first relation between chemistry and current by constructing what we consider the first true battery.
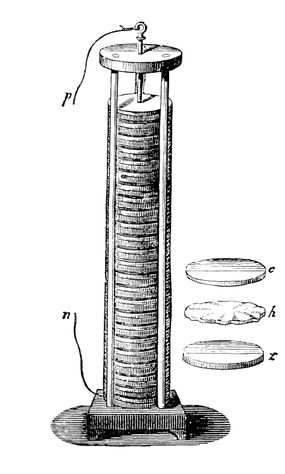
Essentially a voltaic pile, it consisted of alternating layers of zinc and copper separated by brine-soaked cloth. The salty brine functioned as the electrolyte, while the zinc and copper could conduct as the anode and cathode. It provided a steady current and marked the beginning of battery technology. For his contribution, the standard unit of electrical inductance, the volt, is named in Volta’s honor. Our modern understanding of this reaction is known as the Galvanic/Voltaic Cell.
André-Marie Ampère utilized this reaction in his Rotating Conductor Experiment 20 years later, although Ampere was more interested in the interactions of the resulting current that was produced. Still, it was one of the earliest known examples of two half-cell reactions with the oxidation of zinc and the reduction of copper within the same electrolyte, and certainly one of the first that sparked interest in adapting the method for motors and generators.

One of the most significant advancements from there came from John Frederic Daniell in 1836. The Daniell cell used zinc and copper electrodes in separate solutions (zinc sulfate and copper sulfate) connected by a porous barrier or salt bridge. This “half-cell” design solved a key problem of the voltaic pile, the gas buildup on the electrodes that slowed the reaction rate, and his cell produced a more stable current. It became widely used in early telegraph systems, and from that point on, the story of batteries became inseparable from the story of modern technology.
Today, batteries power our world. From clean energy to space exploration, AI to medical devices, our progress depends on our generation’s continuation of these advancements! And it all began from an accident, and the curiosity of innovative, collaborative, and at times, competitive scientists who followed it.
Click here to learn about Ampere’s Two-Wire Experiment!