Magnetizable Materials
Certain materials have certain inherent magnetic properties. Though many materials aren’t magnetic on their own, some are susceptible to magnetization when a magnetic field is applied, yet retain no permanent magnetism (to learn more about materials that do in fact retain magnetic properties, see B-H Curves). The types of magnetization and the orientation of the resulting magnetic field depend on the material that is being magnetized. The three main types of magnetizable materials are diamagnetic, paramagnetic, and ferromagnetic materials. For the purposes of this experiment, we will direct our attention mainly to paramagnetic and diamagnetic materials, focusing heavily on the latter.
Their inherent magnetic properties can be understood from a theoretical standpoint as follows: as a result of the Pauli exclusion principle, which states “ no two identical fermions may occupy the same quantum state in an atom simultaneously”, we know that two electrons (type of fermion) can only occupy the same orbital of an atom if their spins are different (this would mean that their quantum numbers differ slightly and thus they are not in the same state). Conventionally, we state that there are only two possibilities for electron spin: spin up (s=1/2) and spin down (s=-1/2). When two electrons share an orbital, the net spin of that orbital is 0 (1/2 + -1/2 = 0). This case, in which two electrons occupy the same orbital and have a total spin of zero, is the property assigned to diamagnetic electrons.
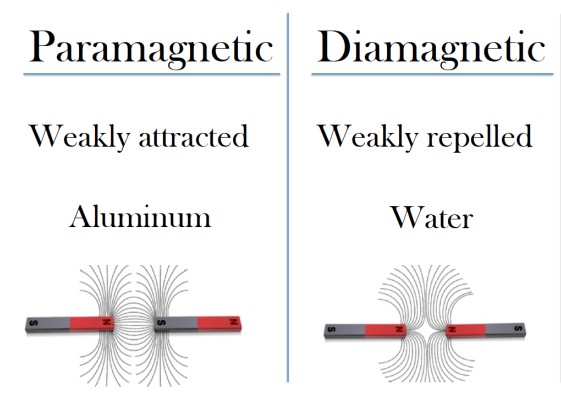
Diamagnetic materials are materials that consist entirely of diamagnetic atoms, which are atoms made up entirely of diamagnetic electron pairs. Diamagnetic atoms repel external magnetic fields because they don’t need to realign their electrons- their orbitals are full. Therefore, diagmagnetic materials, such as water, weakly repel applied magnetic fields.
Contrarily, paramagnetic electrons are unpaired electrons, so when a magnetic field is applied, the unpaired electrons realign in response and are thus attracted to the applied magnetic field. Paramagnetic materials consist of paramagnetic atoms, which are atoms with one or more paramagnetic electrons, so paramagnetic materials, such as aluminum, weakly attract applied magnetic fields. Because thermal energy in the environment randomizes electron spins, none of these materials remain magnetized when the magnetic field is removed.
Diamagnetic Susceptibility
formula
how can we go from diamagnetic susceptibility to force?
ie we know the diamag susc and the field strength, what is the force?
how do we get the graphite to levitate higher?
